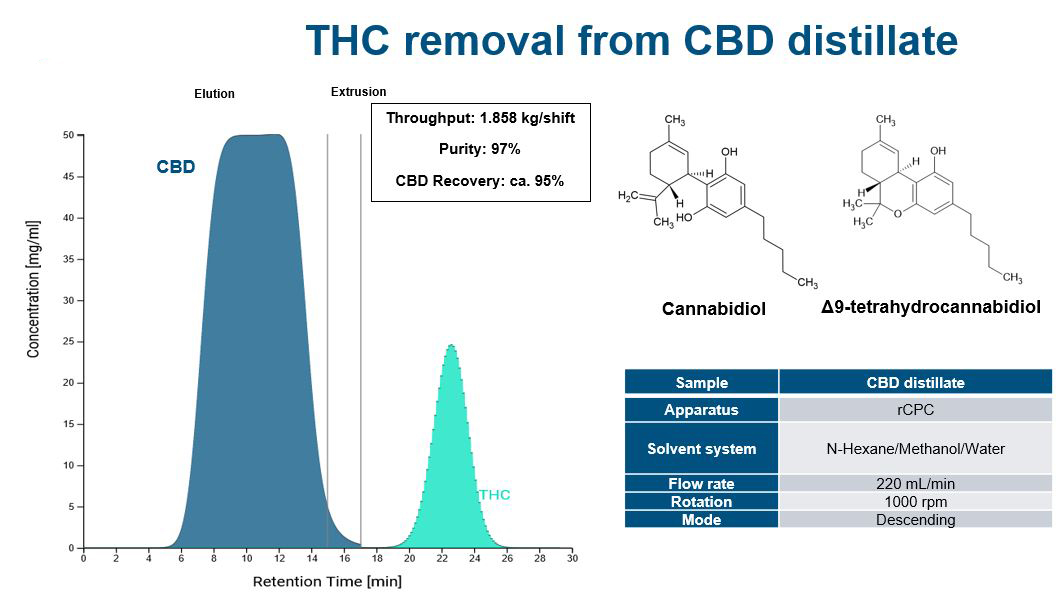
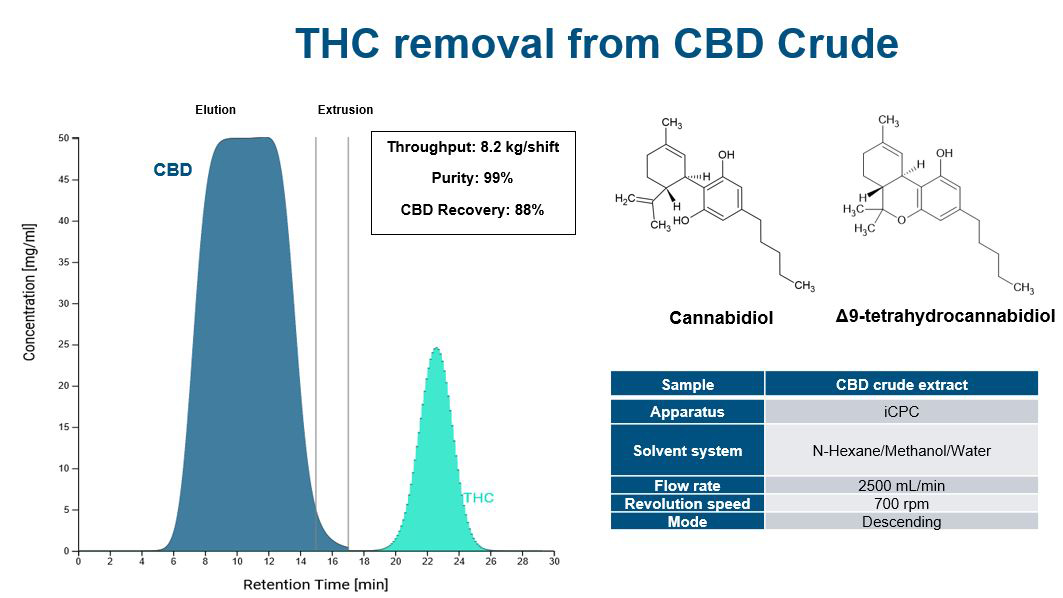
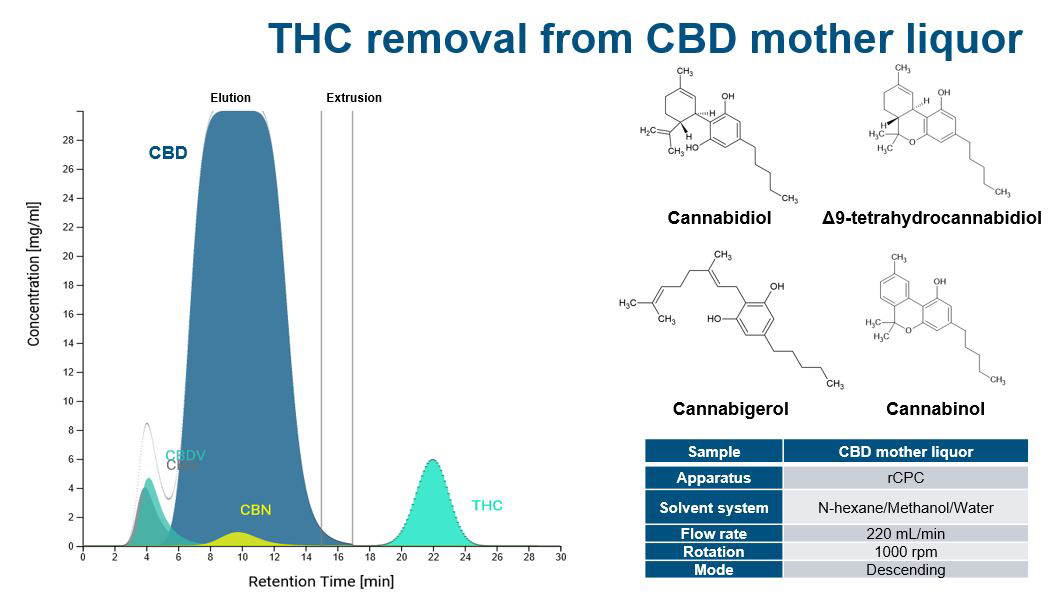
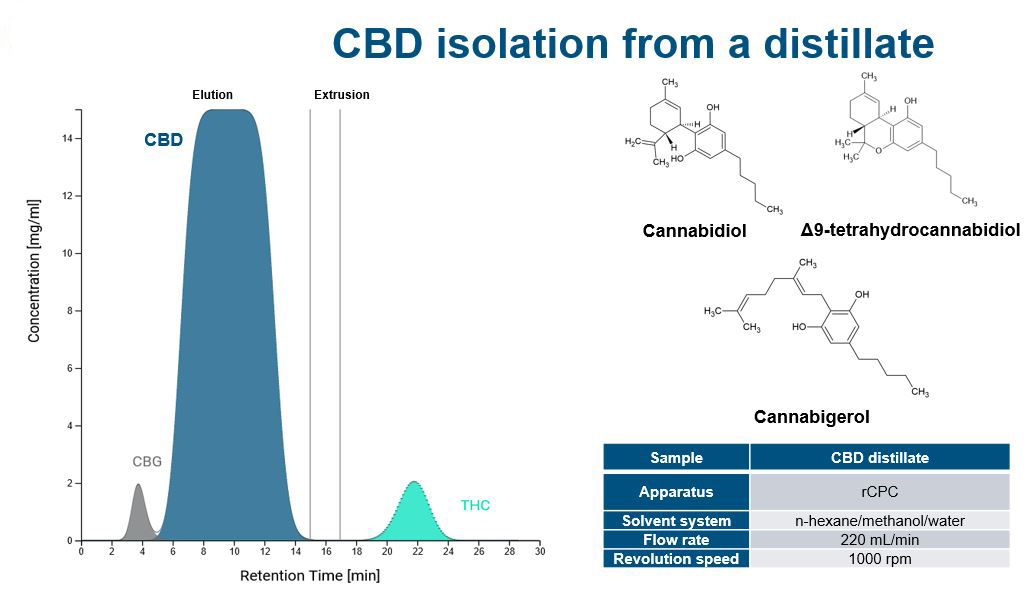
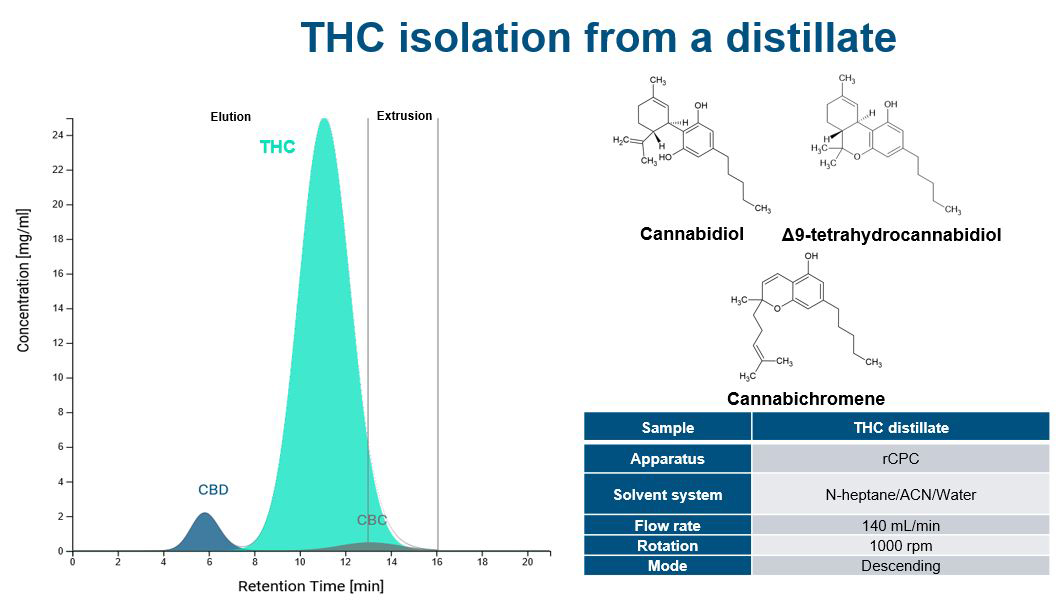
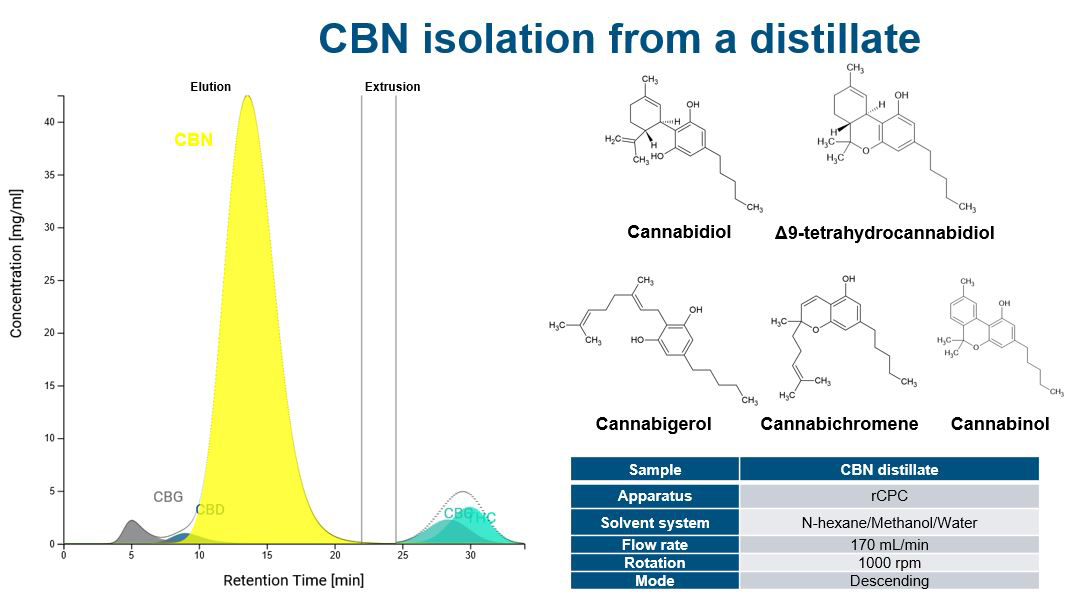
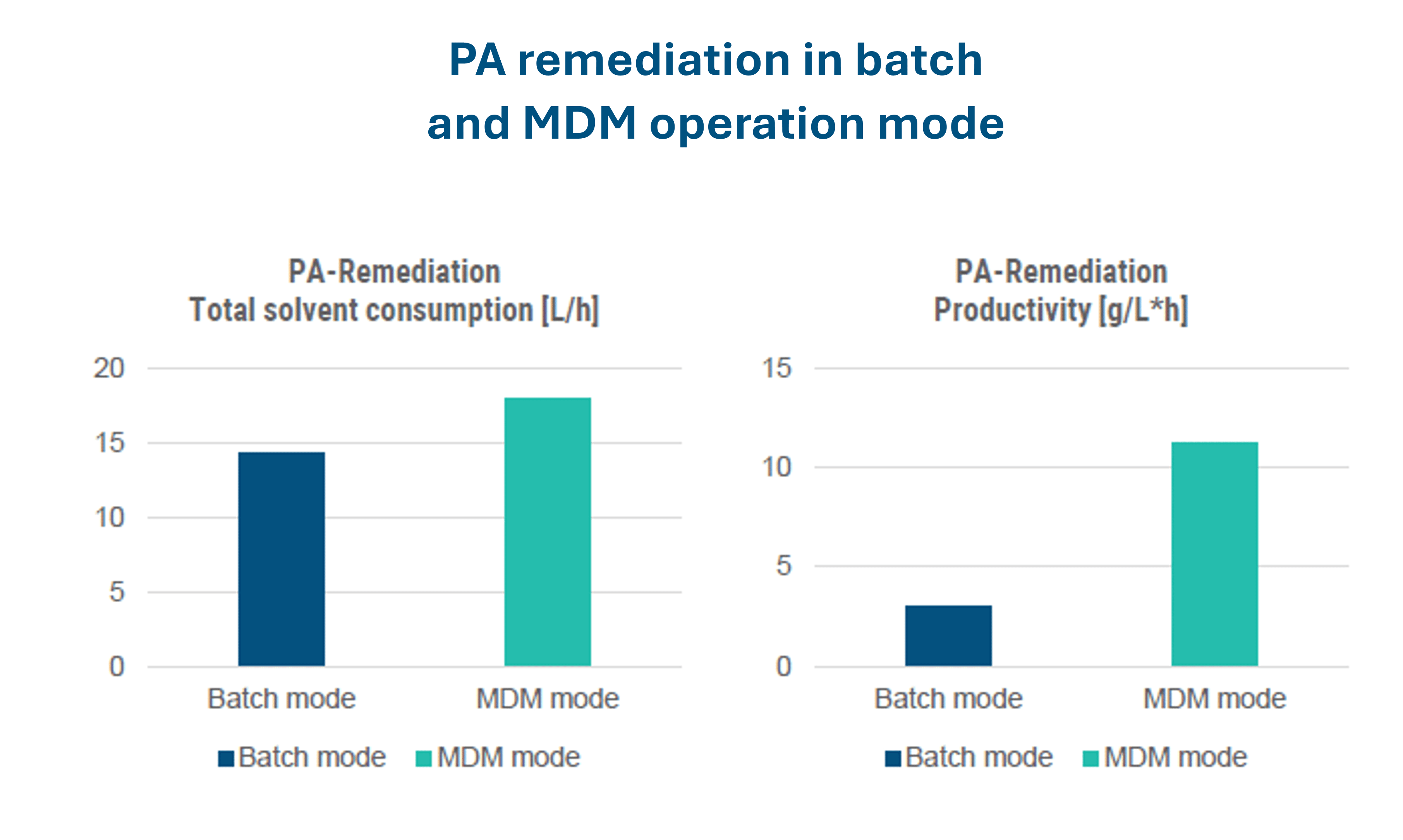
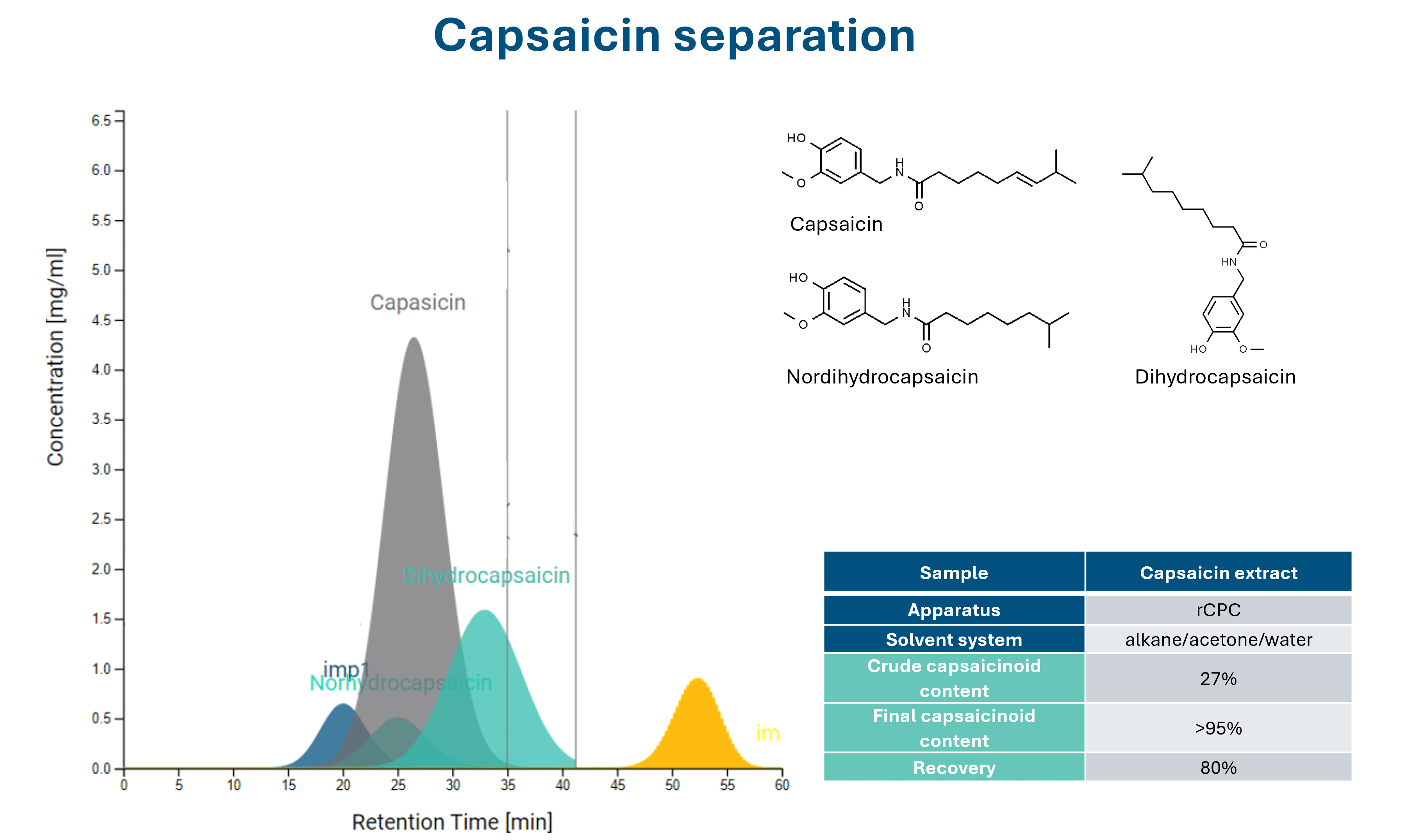
Increasing separation efficiency by pH adjustment in Centrifugal Partition Chromatography
NewsExplore the cutting-edge developments in the Natural Extracts sector! Medicinal plants and botanical resources have historically held significance for their health-enhancing properties, but now, industries are delving deeper into extracting specific phytochemicals for both pharmaceutical and nutraceutical applications. Engage with us as we delve into the realm of purification techniques, particularly chromatography, which is transforming extraction methodologies, yielding pure compounds of immense value. Gain insights into the merits of centrifugal partition chromatography, renowned for its precision in segregating target compounds, and offering versatility in solvent selection. Additionally, uncover its scalability for mass production requirements. Embrace this captivating journey into the future landscape of herbal medicine and natural products!
Download our free white paper here!
Learn how to uncover and maximize hidden treasures in your extraction streams.