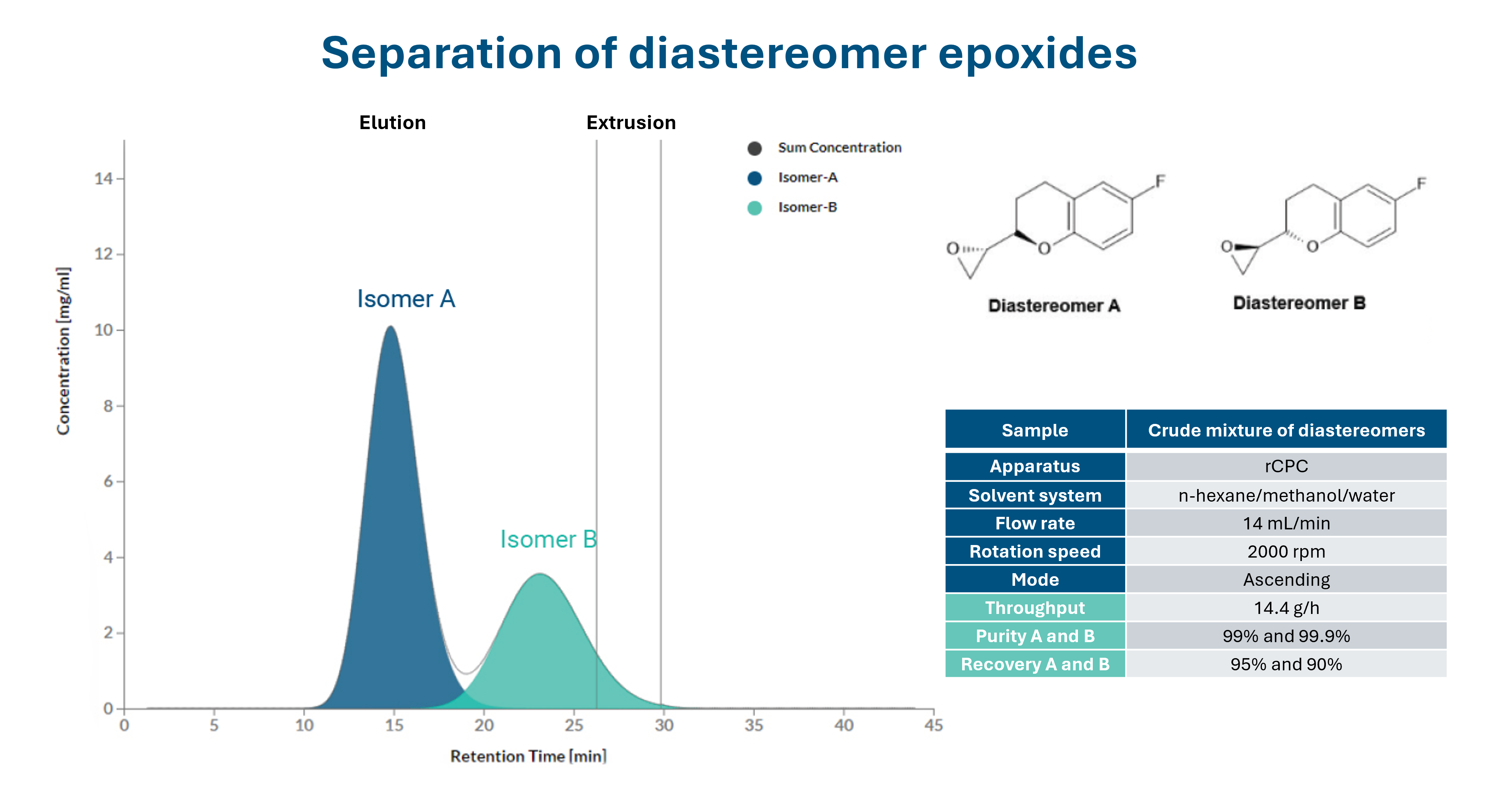
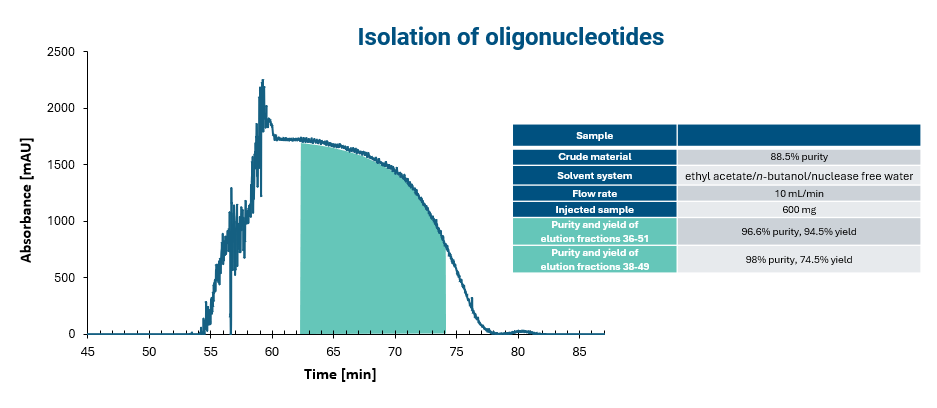
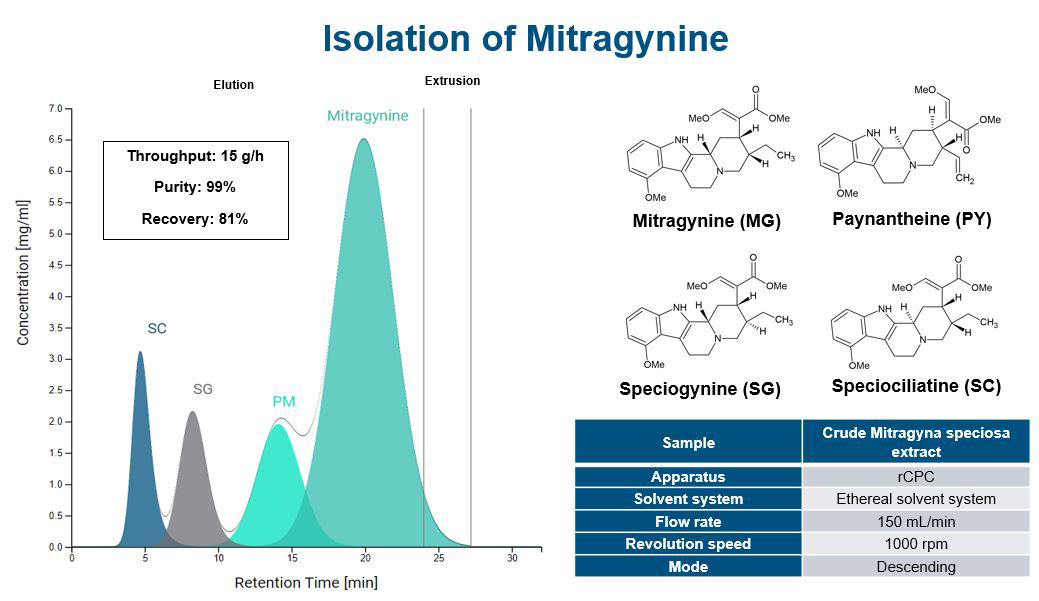
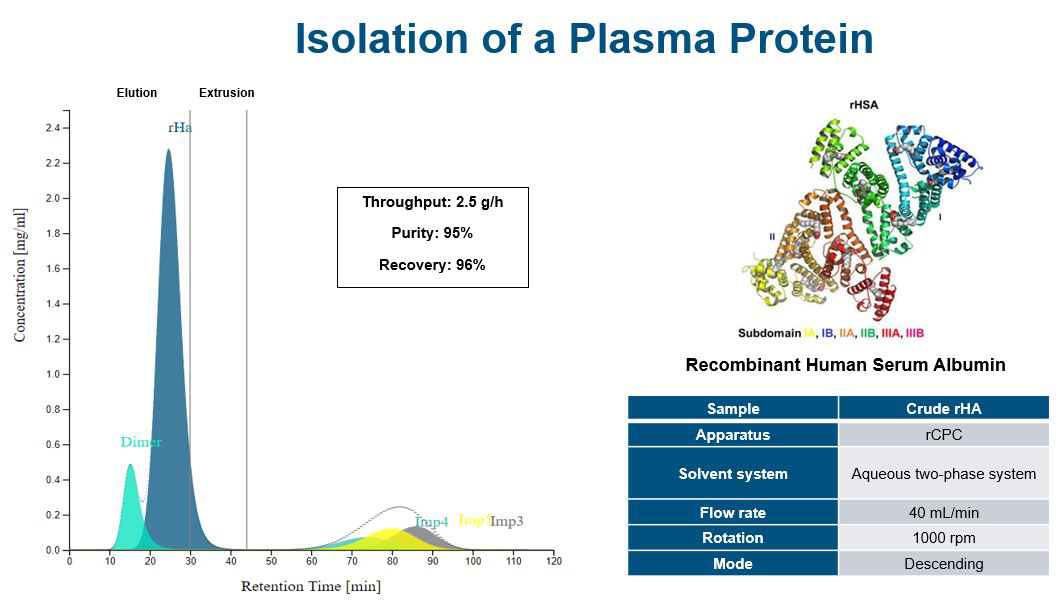
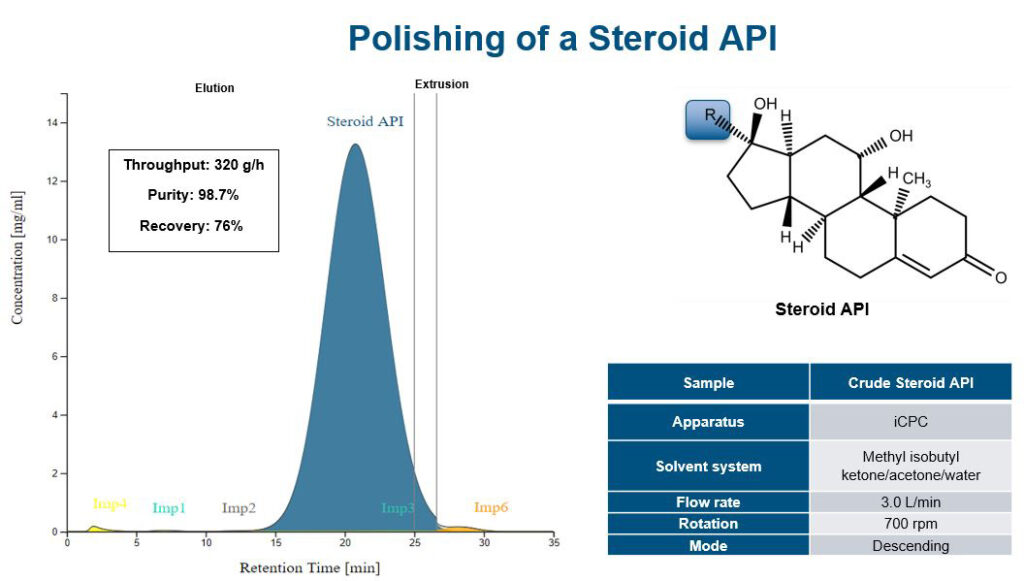
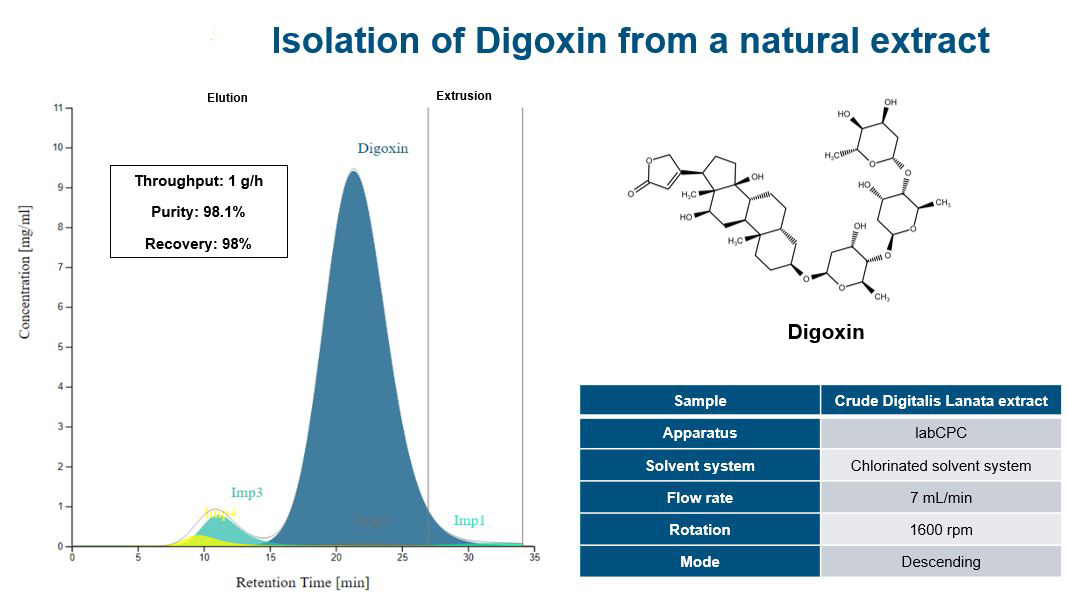
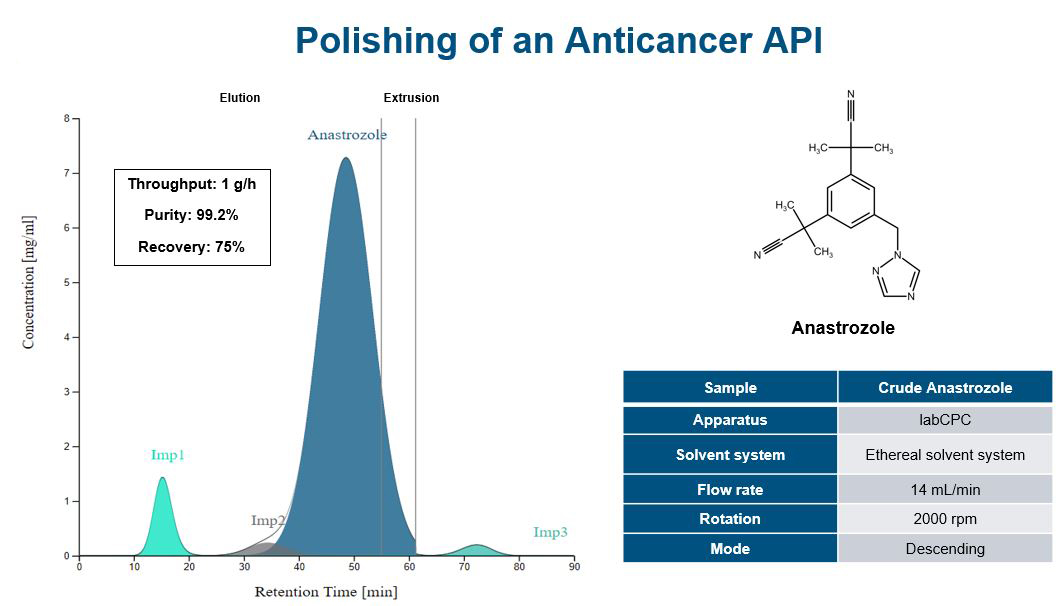
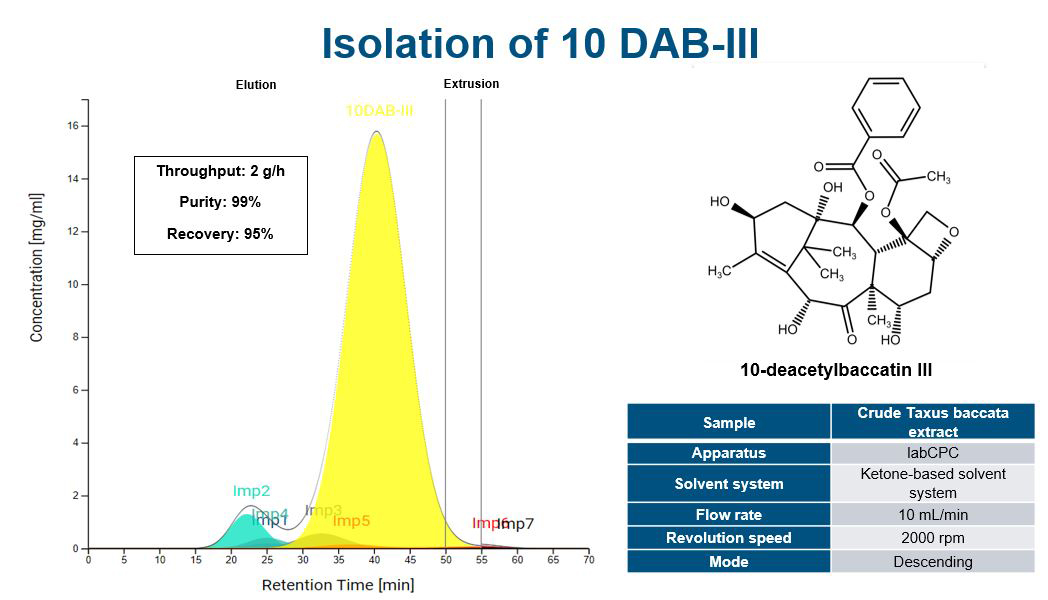
Increasing separation efficiency by pH adjustment in Centrifugal Partition Chromatography
NewsScalable Isomer Separation
Discover the high efficiency and precision that Centrifugal Partition Chromatography (CPC) brings to isomer separation, ensuring the purity and quality of every final product. Separating isomers is challenging in both chemical analysis and industrial production. Traditional methods, like crystallization or distillation, often depend on notable differences in physical properties such as boiling or melting points. However, such disparities are often minimal with isomers, making these methods ineffective.
CPC’s unique advantage lies in its liquid-liquid chromatography technique, which leverages the distinct partition coefficients and solubilities of isomers—fundamental factors that enable CPC to effectively separate complex compounds. This adaptability, along with a wide range of solvent systems, makes CPC an ideal solution for various racemate purification needs. Additionally, it bypasses the need for costly solid stationary phases, reducing operational costs. Pilot- and industrial-scale experiments further demonstrate CPC’s versatility across pharmaceutical production, materials science, agrochemicals, biotechnology, and the food and fragrance industries. Explore our free brochure to learn how CPC can redefine isomer purification for your needs!