Increasing separation efficiency by pH adjustment in Centrifugal Partition Chromatography
NewsSelecting the appropriate stationary and mobile phases is a critical step in chromatographic method development. The primary objective is to achieve effective separations, characterized by sharp, symmetrical, and well-resolved peaks. However, challenges can arise when the stationary phase is suitable, but the mobile phase requires modification—either to ensure proper dissolution of the compound of interest or to enable its separation from other components.
One common issue, when the goal is to separate a component whose charge state is pH-dependent and requires the use of extreme pH values. In this case, adjusting the pH of the mobile phase can be crucial. Unfortunately, this is not always practical for silica-based stationary phases in the long term, as extreme pH conditions can damage the stationary phase over time, leading to reduced separation efficiency.
A potential solution is liquid–liquid chromatography (LC, LLC), including Centrifugal Partition Chromatography (CPC), in which both the mobile and stationary phases are in the liquid state. This approach avoids the degradation issues associated with solid-phase materials and allows for greater flexibility in pH adjustment.
How Chemical Properties Shape Chromatographic Method Design
When designing chromatographic methods, it’s essential to consider the chemical properties of the components to be separated, as these significantly influence retention and separation behavior. A key aspect of this is selectivity, the ability to distinguish between different compounds in a mixture.
Before choosing specific stationary or mobile phases, it is helpful to understand the factors that can influence selectivity. These factors affect the partition coefficient (Kd), which governs how analytes distribute between the stationary and mobile phases:
- Organic Solvent Type: Affects solute-solvent interactions.
- Solvent Strength and Additives: Modulate elution strength and selectivity.
- Stationary Phase Characteristics: Surface properties and functional groups impact retention.
- Temperature: Affects solute volatility and interaction with the stationary phase.
- pH of Mobile Phase: Influences the ionization state of analytes, affecting retention and selectivity.
Once we understand how these parameters affect selectivity, we can make informed decisions about phase selection. The chemical nature of the analyte, such as polarity, charge, or stability, often determines which stationary and mobile phases will be suitable.
Below are examples of recommended stationary and mobile phase combinations for different compound types in solid-liquid chromatography:
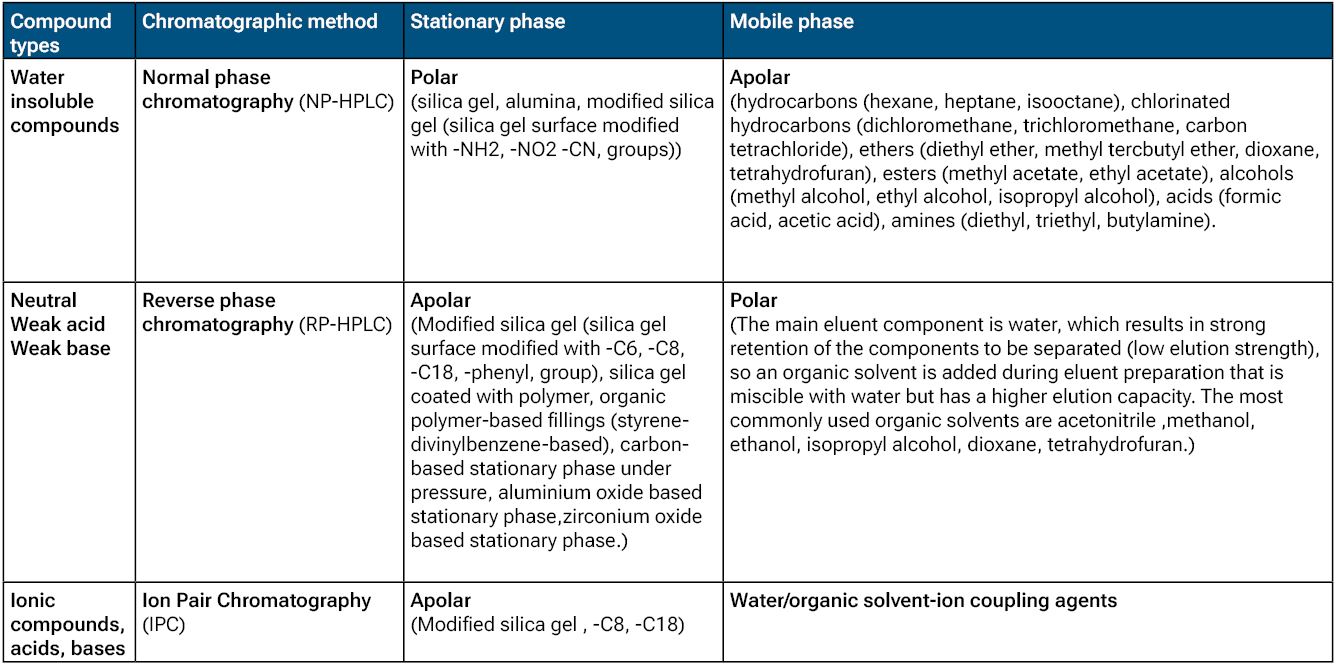
Table 1.: Stationary and mobile phases for HPLC method development
Understanding the role of pH in mobile phase optimization
The pH of the mobile phase can affect chromatography in several ways. Depending on the compound being analyzed, pH can influence selectivity, peak shape, and retention—especially during the purification of compounds whose electric charge depends on the pH value.
For fairly nonpolar or neutral compounds, the effect of pH on resolution and retention is typically minimal. However, for ionizable compounds—such as acids and bases—changes in pH can lead to significant variations in retention factor and selectivity.
When selecting the desired pH, it’s important to consider the dominant ionic form of the analyte. Ideally, all components should be in the same form (either ionized or unionized). The ionization state of a compound relative to the pH of the mobile phase can be predicted using its pKa value. In other words, by adjusting the pH of the mobile phase, we can control the ionization state of the analyte.
Unionized compounds are generally preferred, as they tend to exhibit better retention than their ionized counterparts. Additionally, hydrophobicity must be taken into account—nonpolar compounds typically have longer retention times.
The pKa (acid dissociation constant) is the pH at which half of the molecules of an acid or base are ionized and half are unionized. Although both ionized and nonionized species are present at the pKa, only a single chromatographic peak is observed.
For acids, decreasing the pH increases the proportion of unionized molecules, reducing their overall polarity and leading to longer retention times. In contrast, bases show the opposite response: as pH increases, a larger fraction becomes unionized, which also affects retention time.
Possible drawback of silica-based stationary phase in pH modification of mobile phase
If the solid stationary phase material used in the chromatographic process is silica, problems may arise when using a mobile phase with a modified pH. In this case, it is important to consider the chemistry of the mobile phase, as silica gel is slightly acidic (a polyacid) and soluble in alkaline media. The pH of the mobile phase should generally not exceed the threshold (typically pH 8–9) beyond which silica gel begins to dissolve.
Among modern stationary phases, there are now column packings with improved surface properties that can tolerate mobile phase conditions up to pH 10 without causing silica dissolution. However, low-pH eluents (pH < 1–2) may lead to hydrolysis of alkyl groups bonded to the surface. This hydrolysis alters the polarity of the stationary phase, which can significantly affect the separation mechanism.
Using pH modulation in CPC: A solution for selectivity and overcoming solid-phase limitations
Centrifugal Partition Chromatography (CPC) already has a major advantage over solid-stationary phase chromatography: since both phases are liquids, they can be tailored to suit the specific requirements of the separation process, enhancing selectivity. Selecting the appropriate liquid–liquid system is a crucial step in developing a successful CPC method. Fortunately, a wealth of literature, diagrams, and even apps are available to assist in identifying suitable solvent systems.
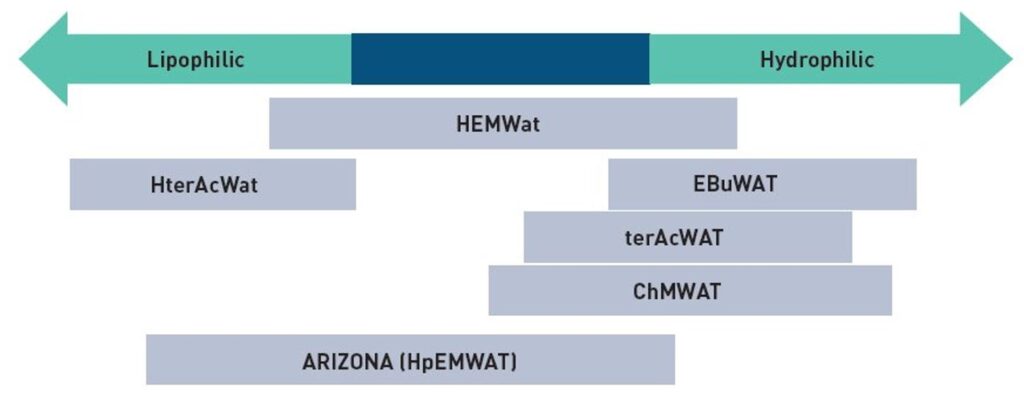
Figure 1. : Polarity map of different solvent systems
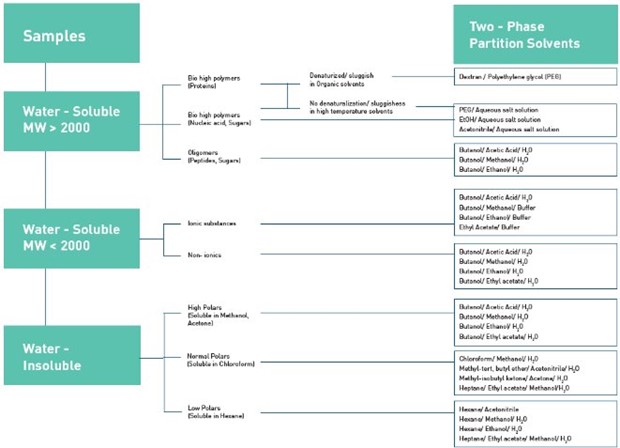
Figure 2. : Solvent system selection algorithm
The composition of the solvent system’s phases can be further modified by adjusting the pH through the addition of different buffers (e.g., citric acid, acetic acid, phosphate buffer) or salts (e.g., sodium chloride solution, ammonium sulfate solution).
The appropriate solvent system is typically selected using the shake-flask method, which determines the partition coefficient (Kd) of the component to be purified. In CPC, separation is based on the differing partition coefficients of the sample components between the mobile and stationary phases. The partition coefficient (Kd) is an equilibrium constant that describes the distribution of a compound between the two liquid phases. For a specific compound of interest (CoI), Kd is defined as the ratio of its concentration in the stationary phase to that in the mobile phase.
During CPC method development, the operating mode can also be chosen—ascending or descending. The ascending mode corresponds to normal-phase HPLC (NP-HPLC), while the descending mode corresponds to reversed-phase HPLC (RP-HPLC).
Unlike traditional chromatography methods that use silica-based stationary phases—which can interact with analytes, causing irreversible adsorption or degradation, especially under extreme pH conditions—CPC uses a liquid stationary phase. This results in improved stability for sensitive compounds.
In CPC, adjusting the pH of the mobile phase to achieve greater selectivity does not carry the same risks as in solid-phase systems. Specifically, there is no concern about irreversible processes such as:
- Degradation of the stationary phase
- Altered retention characteristics
- Loss of functional groups
As a type of liquid–liquid chromatography, CPC benefits from having a liquid stationary phase. This means that even when using mobile phases with very low or high pH (pH 1-14), there is no risk of damaging the stationary phase. As a result, its lifetime, efficiency, and selectivity are preserved. Since there are no physical or chemical changes to a solid surface, the retention time remains stable.
References
- Daniel C. Harris; 2007: Quantitative Chemical Analysis, W.H. Freeman and Company, New York, 2007
- Fekete J. , Kormány R. ,Fekete Sz. (szerk.) Modern folyadékkromatográfia ISBN 978-963-12-8171-2
- Morley, M. Minceva; 2021; Liquid-Liquid Chromatography: Current Design Approaches and Future Pathways, Annual review of Chemical and Biomolecular Engineering, Volume 12